dye laser part 2
Dye preparation
As I don't posses a magnetic stirrer I had to improvise my own solution. My current protocol is as follows:
- Cut in excess leaves of some vegetable (usually a radish or a carrot, it doesn't matter much)
- Put leaves in a beaker
- Pour wanted amount of a solvent, making sure to not exceed 20% of the beaker's volume
- Put on a bass speaker and induce a standing wave in a fluid with high mode number. It's simple to do, I just set generator to 10hz and dial around until I find resonance peak.
Solvents
In order to solve some mysteries, I decided to investigate an influence of the solvent on a dye a bit. I made solutions of dye dissolved in 95% ethanol, 70% ethanol, 99.9% IPA and acetone. Thanks to the Christmas and New Years break, I did it at home and lacked the proper test equipment so the results aren't conclusive. All water-free solvents seemed to behave exactly the same. Dye degraded with a similar speed, a quantum yield wasn't visibly different. On the other hand, 70% ethanol seems to yield a smaller quantum yield, but also slower the degeneration of dye. I'm not sure if a lower degeneration was due to water somehow "helping", or if a lower efficiency simply slowed down the transition to a triplet state (see the next paragraph).
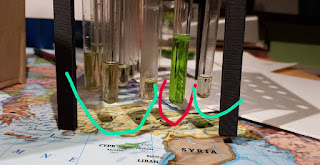 |
| test tubes with chlorophyll samples from different batches |
What was interesting is how depending on solvent evaporation products look visibly different. In cyan are old chlorophyll samples, over 20 days old, allowed to slowly evaporate in the room temperature. Left test tubes have some kind of dots of sides, whereas right one looks like it has very fine particles deposited on its sides.
Dye death
Looking back at this photo, we can see old dyes (cyan) and freshly "cooked" batch, 2 hours old (red). There's visible discoloration of samples and there's a visible decrease in the performance of fluorescence. It's hard to catch on photo using phone and I won't risk an expensive camera near powerful lasers. New batch has a strong red fluorescence, resembling HeNe laser spot on the wall. Old batch has a much fainter color with a yellow discoloration (probably due to green fluorescence).
EDIT
No green fluorescence was emitted. It was just mix of red and diffused blue messing up with eyes.
I have even an older sample, which I left at the University's lab, which is in the basement with shades, with near zero light access. After over 6 weeks and 4 days it looks like 2-3 days old if it was left on windowsill. That lead me to a suspicion that chlorophyll degeneration is not caused by time or heat but by light directly.
 |
| source: https://www.researchgate.net/figure/A-diagram-qualitatively-showing-energy-levels-in-a-chlorophyll-molecule-Transition-from_fig3_235368406 |


Comments
Post a Comment